Designing Universal Influenza Medical Countermeasures
This technology is a universal flu vaccine that aims to prevent and treat seasonal variants of influenza A and B. Therefore, it is relevant to companies in the vaccine and biologics industries.
Researchers
-
identification of variable influenza residues and uses thereof
United States of America | Granted | 12,239,700 -
identification of variable influenza residues and uses thereof
United States of America | Granted | 11,642,407
Figures
Technology
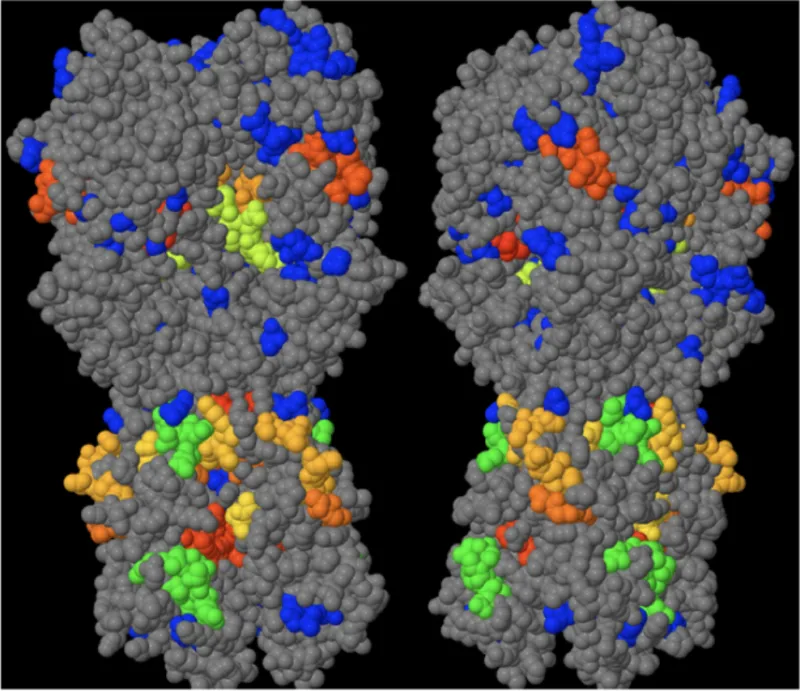
This technology is a prophylactic vaccine to protect against influenza A and B. The vaccine is composed of at least one polypeptide making up an influenza viral protein. Traditionally, the yearly vaccination polypeptides selected contain intact viral surface proteins like hemagglutinin and neuroaminidase. The highly antigenic hypervariable residues are the primary target of the body’s immune response to influenza infections and mutate annually. Non-hypervariable residues are much less prone to annual variation and are common to multiple influenza strains. In this vaccine, hypervariable antigenic amino acid residues in the polypeptides are substituted with non-antigenic amino acid residues that are stable and enable normal protein folding. By triggering B cell activity and antibody production, the vaccine is expected to program the immune system to recognize and protect against most variants of influenza, making it a universal influenza vaccine. A variation of this strategy will enable T-cell responses to cells infected with influenza and could be developed as an alternative or complimentary vaccine.
This technology also enables the selection of surface exposed epitope sites for developing broadly neutralizing (monoclonal) antibody therapeutics (bnAbs). Conserved surface residues are bnAb candidates. Predictions of Ebola virus glycoprotein conserved surface residues in the stem of the protein have been demonstrated to be broadly neutralizing for the three Ebola strains evaluated.
Problem Addressed
While there are effective vaccines available for influenza A and B, the vaccine compositions must be reevaluated yearly to account for new strains emerging due to antigenic shift and drift. This system demands constant analysis of the circulating flu strains each year, and thus relies on imperfect predictions. Since inaccurate predictions lead to vaccine “mismatches,” or vaccines that protect against strains other than the ones circulating, there is a need for a vaccine that can effectively target various influenza strains more broadly, for either generating an immunogenic response as a vaccine or prompting the production of antibodies as a therapeutic.
Vaccination induced immune responses are specific to the selected strains, with antibodies targeting highly antigenic surface exposed hypervariable residues. The virus readily mutates these residues to escape immune responses with antigenic variation. Immune responses to highly antigenic hypervariable residues drives the need for yearly vaccinations.
Advantages
- Can be used for both protection against and treatment for influenza, unlike current flu vaccines
- Applies to a variety of Influenza A and B strains without relying on constant surveillance of circulating strains, saving time and resources
Publications
Darrell O. Ricke, and Robert W. Malone. Medical Countermeasures Analysis of 2019-nCoV and Vaccine Risks for Antibody-Dependent Enhancement (ADE). Preprint. 2020 March 8. doi: 10.20944/preprints202003.0138.v1
Darrell O. Ricke, and Anna Shcherbina. Dawn: Rapid large-scale protein multiple sequence alignment and conservation analysis. IEEE. 2015 September 15-17. doi: 10.1109/HPEC.2015.7322463
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.