Mucin-inspired Glycopolymers Can Selectively Kill Vaginal Pathogen Gardnerella Vaginalis Without Impacting Growth of Beneficial Lactobacillus Crispatus
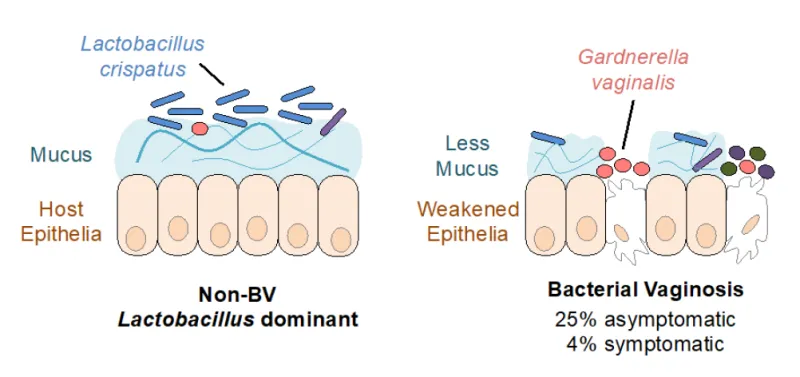
This technology uses mucins and mucin-inspired glycopolymers to destroy biofilms and reduce pathogenic Gardnerella Vaginalis bacteria commonly found during bacterial vaginosis (overgrowth of bacteria) in the vagina while not harming beneficial bacteria, such as Lactobacillus Crispatus.
Researchers
-
methods and compositions for treating or preventing a vaginal infection of gardnerella vaginalis
United States of America | Published application -
methods and compositions for treating or preventing a vaginal infection of gardnerella vaginalis
European Patent Convention | Published application
Figures
Technology
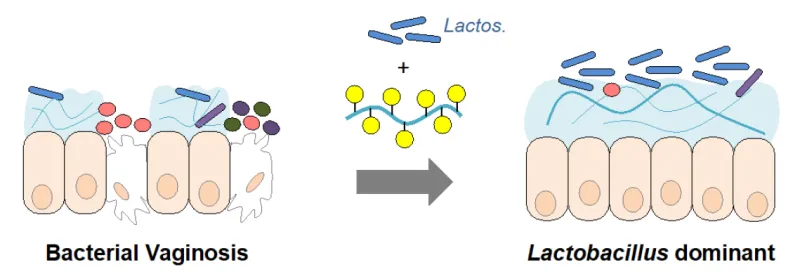
This technology uses mucin-inspired glycopolymers, polymers mimicking certain properties of mucus, to alter the microbial environment and reduce growth of pathogenic G. vaginalis. Certain sugar molecules of mucins, such as galactose, act as signaling molecules to selectively trigger a reduction in pathogenic behavior, and beneficial bacteria are not harmed in the process. To create the mucin-inspired glycopolymers, galactose is grafted onto silk fibroin or norbornene forming a synthetic mucus. Presence of the galactose sugar molecule leads to reduced biofilms, less pathogenic bacterial growth, and decreased endocervical cell death. The mucin-inspired glycopolymers can also be coupled with probiotic treatment to further help in rebalancing the microbiome.
Problem Addressed
Bacterial vaginosis (BV) is an imbalance in bacterial growth leading to an increased number of pathogenic bacteria such as Gardnerella vaginalis (G. vaginalis). BV is associated with an increased risk of acquiring sexually transmitted infections and with adverse pregnancy outcomes. Currently, the most common treatment is broad spectrum antibiotics via oral or topical application, which over time contributes to antibiotic resistance. Furthermore, the antibiotic usage can itself create an imbalance in the body’s microbiome, which can increase susceptibility to other infections and complications. A vaginal microbiome transplant is an alternative, but donors are limited, and the procedure is more expensive than broad spectrum antibiotics. The mucin-inspired glycopolymers of this invention avoid disrupting the microbiome’s balance, while subduing the overgrowth of G. vaginalis for less than a microbiome transplant.
Advantages
- Avoids the use of antibiotics and thus complications due to good bacteria being killed indiscriminately.
- Selectively reduces pathogenic bacteria, such as G. vaginalis.
- Can be coupled with probiotic treatments.
- Cheaper, easier alternative than vaginal microbiome transplants.
- No wait time for a microbiome donor.
Publications
Takagi, J., Aoki, K., Turner, B.S. et al. Mucin O-glycans are natural inhibitors of Candida albicans pathogenicity. Nat Chem Biol 18, 762–773 (2022). https://doi.org/10.1038/s41589-022-01035-1
Wang, B.X., Wheeler, K.M., Cady, K.C., Lehoux, S., Cummings, R.D., Laub, M.T., Ribbeck, K. (2021). "Mucin Glycans Signal through the Sensor Kinase RetS to Inhibit Virulence-Associated Traits in Pseudomonas aeruginosa." Current Biology, Elsevier, 11 January 2021. doi: https://doi.org/10.1016/j.cub.2020.09.088
Wheeler, K.M., Cárcamo-Oyarce, G., Turner, B.S. et al. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat Microbiol 4, 2146–2154 (2019). https://doi.org/10.1038/s41564-019-0581-8
Kavanaugh, N.L., Zhang, A.Q., Nobile, C.J., Johnson, A.D., Ribbeck, K. (2014). "Mucins Suppress Virulence Traits of Candida albicans." mBio, 11 November 2014. doi: https://doi.org/10.1128/mbio.01911-14
Caldara, M., Friedlander, R.S., Kavanaugh, N.L., Aizenberg, J., Foster, K.R., Ribbeck, K. (2012). "Mucin Biopolymers Prevent Bacterial Aggregation by Retaining Cells in the Free-Swimming State." Published in Current Biology on November 08, 2012. doi: https://doi.org/10.1016/j.cub.2012.10.028
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.