Electrochemically Responsive Heterogeneous Catalysis Using Redox Polymer/Conducting Fibers Hybrids
This technology has applications in the control of chemical reactions, such as the Michael addition reactions used to synthesize steroids and other organic compounds, with redox-switchable catalysts.
Researchers
-
electrochemically responsive composites
of redox polymers and conducting fibers
Patent Cooperation Treaty | Published application
Figures
Technology
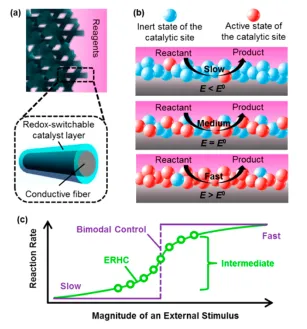
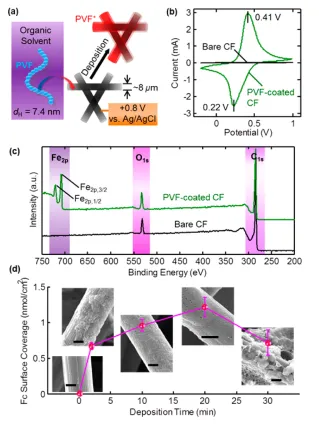
The electrochemically responsive heterogeneous catalyst described in this invention is made up of an electrically conductive matrix and a conformal coating containing an electrochemically active catalytic molecule. For example, a heterogeneous catalyst for the Michael addition of methyl vinyl ketone (MVK) and ethyl-2-oxycyclopentane carboxylate (E2OC) -- a reaction used in steroid synthesis -- can be made by coating a porous carbon fiber matrix with polyvinylferrocene (PVF). Ferrocene (Fc) has no catalytic activity, per se, but its oxidized form, ferrocenium (Fc+), catalyzes the addition reaction.
To control the rate of the reaction, a voltage is applied to the carbon fiber matrix to create a localized oxidative environment, which in turn oxidizes Fc into Fc+ to increase catalytic activity. The proportion of Fc to Fc+ depends on the magnitude of the voltage applied, allowing the reaction rate to be continuously modulated over a range. The additional flexibility this method provides over binary-switching catalytic schemes is useful in situations including selectivity control in complex reaction networks or thermal regulation of exothermic reactions. Since this responsive catalysis system relies on redox switching of the catalytic compounds instead of structural changes to the matrix, it maintains a constant volume and is resistant to catalyst leaching, making it well-suited for use in fixed-bed flow reactors.
Problem Addressed
The ability to control the rate of chemical reactions is a critical aspect of reaction engineering. Since many important reactions used in chemical synthesis involve heterogeneous catalysis, a popular strategy to controlling their kinetics is to manipulate the efficacy of the catalysts. Conventionally, this is achieved by changing either the concentration or accessibility of catalytic species by inducing morphological changes to the catalyst carrier using chemical or thermal stimuli. These methods have two limitations. First, the inherent tendency of chemical and thermal inputs to propagate through diffusion prevents fine-grained spatial control of catalytic activity. Secondly, morphological changes to catalyst carriers often entail volumetric and phase (sol-gel) transitions that make existing responsive catalysis strategies challenging to implement in fixed-bed flow reactors. The novel class of responsive heterogeneous catalysts described in this invention addresses these problems by responding to highly localizable electrochemical stimuli and relying on redox transitions instead of morphological changes to control catalytic activity.
Advantages
- Fixed catalyst volume and no leaching allows use in fixed-bed flow reactors
- Temporal as well as spatial control of reaction kinetics
- Allows continuous modulation of reaction rate
Publications
"Electrochemically Responsive Heterogeneous Catalysis for Controlling Reaction Kinetics," J. Am. Chem. Soc. (2015).
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.