Electrochemical Carbon Dioxide Capture System
The capture and release of CO2 from a gas mixture, particularly a flue gas, has applications in several contexts, ranging from carbon capture and sequestration for coal-fired power plants, to life-support systems in spacecraft and submarines.
Researchers
-
methods and systems for carrying out a ph-influenced chemical and/or biological reaction
United States of America | Granted | 9,567,678 -
methods and systems for carrying out a ph-influenced chemical and/or biological reaction
United States of America | Granted | 10,610,824
Figures
Technology
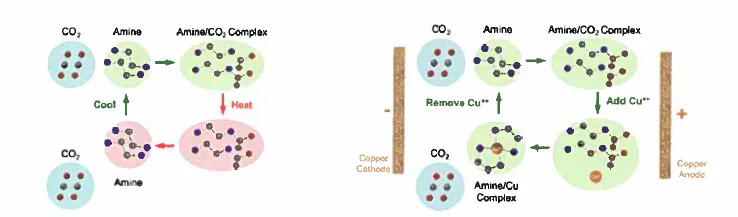
The electrochemical amine regeneration (EMAR) process takes place in a cell with two copper chambers that contains a positively-charged anode in one and a negatively-charged cathode in the other. The CO2–amine molecules enter the first chamber in an aqueous solution and come into contact with copper ions released by the charged anode. The copper reacts with the amine, releasing the CO2 in the process. The amine-copper complex then travels to the other chamber where the cathode strips away the copper ions, leaving the amine molecules free to once again capture CO2.
Instead of depositing and dissolving the electrode material from a surface comprised of the same material, the system is designed such that the metal ions are inserted into and out of a porous electrode material. For example, carbonaceous materials (e.g., carbon nanotubes, nanofibers, graphite) can be used as insertion/deinsertion materials for the copper ions. Using such insertion/deinsertion hosts significantly improves the cycling stability of the system for the electrochemical carbon dioxide capture of CO2.
Problem Addressed
CO2 capture is the prerequisite step in CO2 sequestration, a process of transporting waste CO2 and depositing it into storage sites where it cannot enter the atmosphere. This process aims to mitigate the effect of fossil fuels on global warming and ocean acidification. Current CO2 capture technology relies on a thermal cycle, wherein a solvent (usually an amine solution) that can complex dissolved CO2 is heated after capture in a stripper to force the release of gas. That process is energetically inefficient and prohibitively expensive due to large capital investments needed to modify existing power stations.
The inventors have developed an alternative process that relies on a voltage cycle, wherein metal ions generated at the anode side of an electrochemical cell strongly chelate the amine solvent and release CO2. On the cathode side of the cell, the metal ions are redeposited on the electrode to regenerate the amine solvent.
Advantages
- EMAR system can be used in steam-free environments including cement, steel and aluminum manufacturing plants, which account for 10% of global CO2emissions
- System requires only electricity to run, making it easy to retrofit in existing facilities
- Removes even low concentrations of CO2 in confined spaces (e.g., submarine, space shuttle)
Publications
Eltayeb, Aly O., et al. "Energetics of Electrochemically-mediated Amine Regeneration." Energy Procedia 63 (2014): 595-604. ISSN 1876-6102. https://doi.org/10.1016/j.egypro.2014.11.064.
Sterna, Michael C., and T. Alan Hatton. "Bench-scale demonstration of CO2 capture with electrochemically-mediated amine regeneration." RSC Advances, 4 (2014): 5906-5914. DOI: 10.1039/C3RA46774K.
Stauffer, Nancy W. "A New Way to Capture CO2 Emissions: Lower Costs, Easier Installation." Published on June 10, 2014.
Stern, Michael C., Fritz Simeon, Howard Herzog, and T. Alan Hatton. "Post-combustion Carbon Dioxide Capture Using Electrochemically Mediated Amine Regeneration." Energy & Environmental Science, 2013, 6, 2505-2517. doi: 10.1039/C3EE41165F. Published on June 6, 2013.
Stern, Michael C., Fritz Simeon, Howard Herzog, and T. Alan Hatton. "An Electrochemically-mediated Gas Separation Process for Carbon Abatement." Energy Procedia, 2013, Volume 37, Pages 1172-1179. ISSN 1876-6102. doi: 10.1016/j.egypro.2013.05.214.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.