Pooled Screening Identifies Genomic Features (Biomarkers) Modulating Nanoparticle-Cell Interactions
This invention is the first biomarker that is both nanoparticle-specific and cancer type-agnostic and can be used for identifying cancer patients who are well-suited for nanoparticle-based therapy. Therapeutic applications of this invention could include precision medicine fields of oncology, immunotherapy, and genome engineering.
Researchers
-
solute carrier family 46 member 3 (slc46a3) as marker for lipid-based nanoparticle cancer therapy and diagnostics
United States of America | Granted | 12,194,055
Figures
Technology
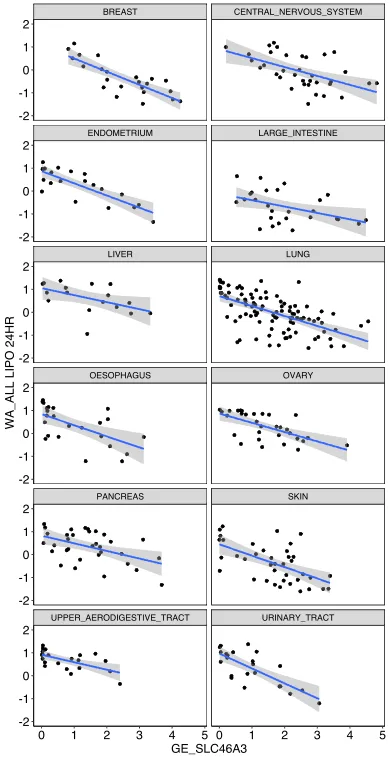
The invention covers a lysosomal membrane protein SLC46A3 that acts as a nanoparticle-specific biomarker for informing cancer cell affinity for liposomal nanoparticles. This discovery was derived from a high-throughput pooled screen of hundreds of cancer cell lines against 40 nanoparticle and antibody formulations - low SLC46A3 expression showed strong association with high liposomal nanoparticle uptake. This inverse relationship is consistent across multiple cancer cell types and lineages as well as liposomal nano-formulations, thus establishing SLC46A3 expression as a cancer-agnostic biomarker. This relationship was validated in vivo via intravenous and intratumoral injection of a liposomal nanoparticle formulation using an engineered melanoma mouse model. Notably, in patient tumor data, cancer cells expressing low levels of SLC46A3 are associated with aggressive tumor phenotypes and poor prognosis. Therefore, strategic integration of this biomarker into patient cohort selection during clinical trials could potentially minimize non-uniform biological response to nanoparticles, thereby providing a more uniform clinical readout of the investigational drug.
Problem Addressed
Nanoparticles have gained momentum in the world of nanomedicine but face challenges in translation to clinical practice. Today, nanoparticles are the go-to delivery vehicles for a broad range of therapeutic modalities – from chemotherapeutic agents to CRISPR-based drug complexes and mRNA vaccines. However there are only a handful of FDA-approved nanoparticle-based medicines. This lack of clinical progress can be attributed to sub-optimized designs, formulations, and non-uniform clinical responses in patients. Engineering efforts are poised to tackle nanoparticle optimization challenges but are insufficient to address the lack of uniform clinical efficacy. As one can imagine, screening nanoparticle efficacy across a vast heterogeneity of biological barriers in a broad population could mask nanoparticle potential in treating a slightly smaller subset of patients. Therefore, selecting the right patient group can aid in accelerating clinical progress of nanoparticle technology.
Current clinical trial designs do not stratify patients based on their biological affinity to liposomal nanoparticles due to absence of informative biomarkers. The inventors take a data-driven approach to identify the first nanoparticle biomarker that can aid in making such decision to improve clinical efficacy outcome.
Advantages
• Enable prediction of cancer cell affinity to liposomal nanoparticles
• Applicable to multiple nanoparticle formulations
• Enable identification of cancer patient cohorts likely to respond favorably to nanotherapeutics
• Effective trafficking of liposomal nanoparticles to aggressive tumors with low SLC46A3 expression
• Potentially expandable to disease indications beyond oncology
• Potentially applicable to additional kinds of nanoparticles
Publications
Natalie Boehnke, Joelle P. Straehla, Hannah C. Safford, et al. Massively parallel pooled screening reveals genomic determinants of nanoparticle-cell interactions. bioRxiv. 2021 Sept 24. doi: 10.1101/2021.04.05.438521
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.