Polymers for Selective Heavy Metal Removal
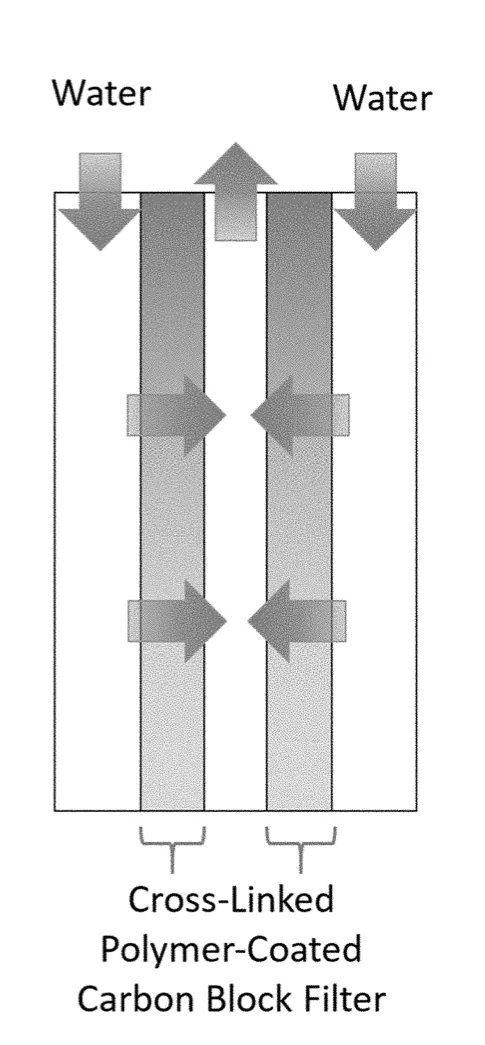
The invented polymers are designed for water treatment where there are collections of toxic metals. Used in conjunction with gravity filtration or a carbon block filter, water can be cleansed of both particulate matter and dissolved heavy metals.
Researchers
-
polymers for selective heavy metal removal
Patent Cooperation Treaty | Published application -
polymers for selective heavy metal removal
United States of America | Granted | 11,746,191
Figures
Technology
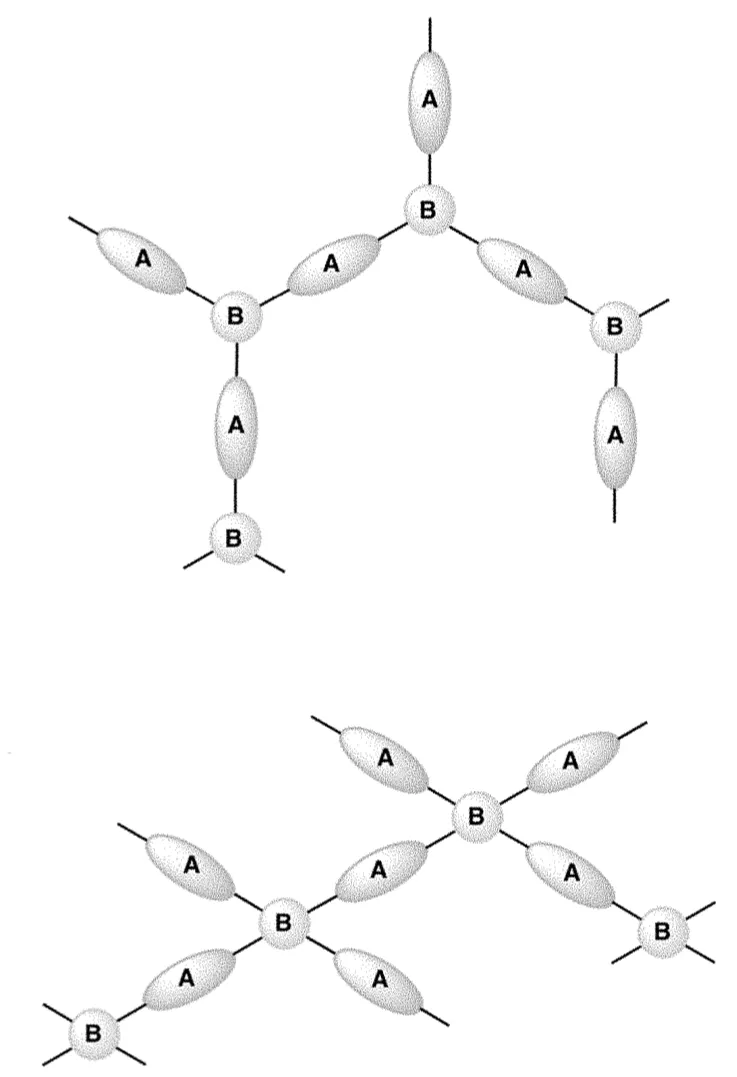
This cross-linked polymer network captures high volumes of heavy metal ions through soft-soft interactions. These interactions result from the high sulfur content in the branching monomers. Sulfur-containing chains have a high degree of cross-linking which prevents the network from falling apart and ensures no leaching of organics into the water. The network is also designed to have mesoporosity or macroporosity for a high contact surface area with heavy metals, enabling a high rate of filtration. Included in this technology are synthesis methods that create a tri-branched or tetra-branched cross-linker based structure.
Problem Addressed
Heavy metal contamination in water is a major problem in both developed and developing countries. Even low concentrations of mercury and lead pose a serious threat to human health. Past approaches to heavy metal filtration, like ion exchange resins, are either ineffective or highly expensive. A highly efficient, scalable technology with minimal energetic needs for removing heavy metal ions from water would be ideal.
Advantages
- More than 100-fold reduction in Lead concentration from 150 ppb to less than 1 ppb
- Selectively chooses heavy metals like Mercury and Lead over common natural ions
- Network structure allows dissolution of high concentrations of ions while keeping structural integrity
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.