Diazene Directed Modular Synthesis of Oligocyclotryptamines
This technology is a novel synthesis technique for making oligocyclotryptamine molecules with applications in chemical engineering and pharmaceutical R&D.
Researchers
-
diazene directed modular synthesis of compounds with quaternary carbon centers
United States of America | Granted | 10,640,508
Figures
Technology
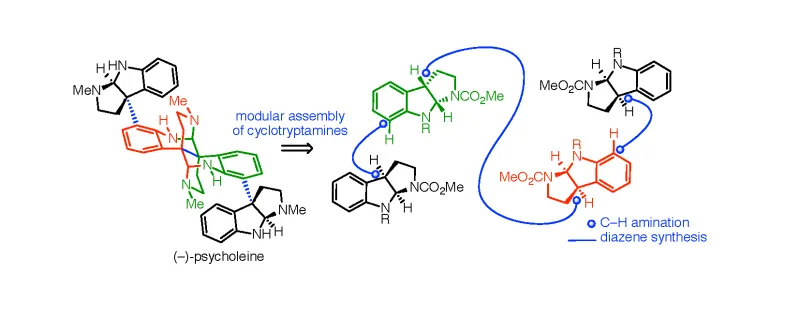
This synthesis reaction is the first generalizable strategy for generating oligocyclotryptamines with complete stereochemical control. The reaction is facilitated by first synthesizing aryl-alkyl diazenes that serve as monomers that are then iteratively reacted to build the large oligocyclotryptamine molecules. Additionally, this system is modular, and the inventors describe successful synthesis of pure enantiomeric forms of five different oligocyclotryptamine molecules by simply switching diazene monomer choice.
Problem Addressed
Hexahydropyrroloindole alkaloids, including oligocyclotryptamines, are structurally complex, naturally occurring chemicals with a wide range of therapeutically relevant properties, including antibacterial, antifugal, anticancer, and analgesic activity. However, the biological activity of oligocyclotryptamines is highly dependent on the enantiomeric form of the molecule, and the rare oligocyclotryptamines found in nature exist as a mix of enantiomers. Additionally, the presence of multiple stereocenters makes chemical synthesis of pure enantiomers nearly impossible, and therefore, the potential therapeutic properties of oligocyclotryptamines have remained largely unexplored.
Advantages
- Efficient synthesis of biologically active oligocyclotryptamines
- Complete control of stereochemistry at all stereocenters
- Highly modular, generalizable synthesis that can be applied to synthesis of many different oligocyclotryptamine molecules
Publications
Lindovska, Petra, and Mohammad Movassaghi. "Concise Synthesis of (−)-Hodgkinsine, (−)-Calycosidine, (−)-Hodgkinsine B, (−)-Quadrigemine C, and (−)-Psycholeine via Convergent and Directed Modular Assembly of Cyclotryptamines." Journal of the American Chemical Society, 139 (2017): 17590-17596. doi: 10.1021/jacs.7b09929.
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.