Method for Producing Phosphorus Chemicals from Wet Process Phosphate
Processed phosphorus has applications in the agricultural, battery, flame retardant, pharmaceutical, plastics, and electronics industries. Methods disclosed by this invention allow for cheaper and cleaner production of useful phosphorous compounds.
Researchers
-
method for producing phosphorus chemicals from wet process phosphate
United States of America | Granted | 11,993,623
Figures
Technology
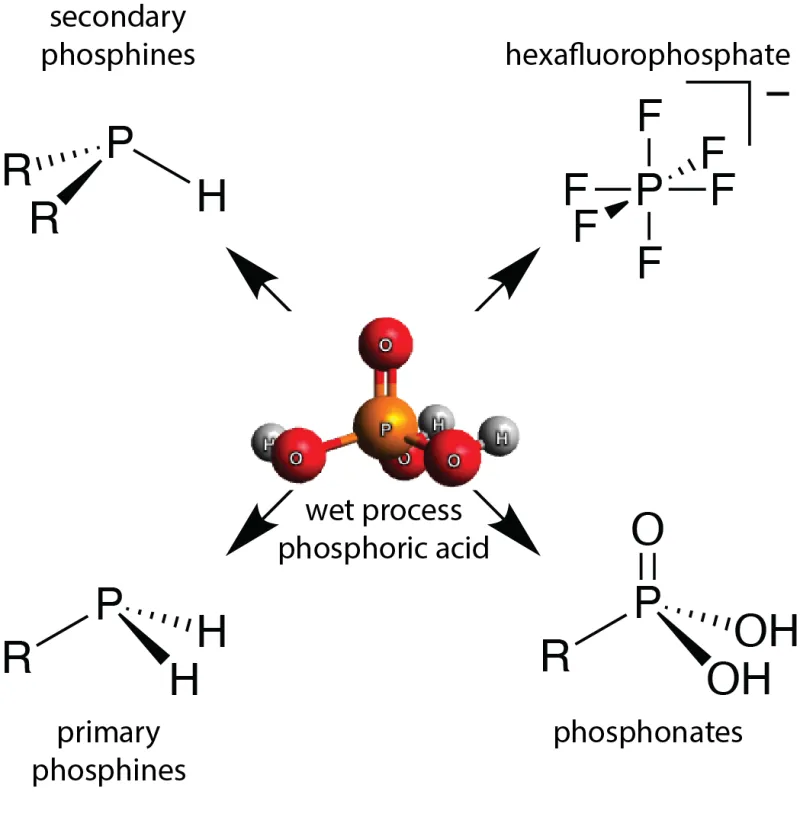
The method disclosed by this invention uses trichlorosilane, an inexpensive high production volume chemical used in the semiconductor and solar photovoltaic industries, to produce from phosphoric acid a new phosphorus-containing reagent, tetrabutylammonium bis(trichlorosilyl)phosphide. This silylphosphide salt and related reagents can serve as an intermediate to compounds traditionally derived in industrial settings from white phosphorus via processes also disclosed by this invention. Accessible compounds include primary and secondary alkyl phosphines, phosphonates, and hexafluorophosphates.
Problem Addressed
Numerous phosphorous-containing compounds essential to modern industry are derived from white phosphorous. This extraordinarily hazardous material is produced from phosphate rock by the “thermal process,” a heavily polluting method that generates large volumes of carbon monoxide and carbon dioxide. Phosphate minerals can also be processed by the cheaper and comparatively clean “wet process” to produce the phosphoric acid used in fertilizers, but historically there have not been methods for converting this phosphoric acid into high value fine chemicals. A method to convert wet process phosphoric acid into the fine chemicals required by the battery, flame retardant, pharmaceutical, plastics, and electronics industries would both green the processes and avoid hazardous intermediates.
Advantages
-
Cheaper and cleaner than traditional thermal process white phosphorous production
-
Avoids extremely hazardous intermediates
-
Utilizes starting materials already available at scale (wet process phosphoric acid and trichlorosilane)
Publications
Michael B. Geeson, et al. Orthophosphate and Sulfate Utilization for C–E (E = P, S) Bond Formation via Trichlorosilyl Phosphide and Sulfide Anions. Journal of the American Chemical Society. 2019 Mar 22. doi: 10.1021/jacs.9b01475
Michael B. Geeson and Christopher C. Cummins. Phosphoric Acid as a Precursor to Chemicals Traditionally Synthesized from White Phosphorus. Science. 2018 Feb 8. doi: 10.1126/science.aar6620
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.