Model-based Control for Column-based Continuous Viral Inactivation of Biopharmaceuticals
This invention is a low-cost, column-based viral inactivation system for use in continuous manufacturing of biologics, with applications in pharmaceutical development and manufacturing.
Researchers
-
model-based control for column-based continuous viral
inactivation of biopharmaceuticals
United States of America | Published application
Figures
Technology
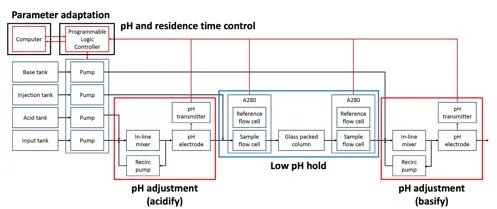
This invention is a low-cost, column-based viral inactivation system for use in continuous manufacturing of biologics. Notably, this system allows for precise control over the pH and residence time, which are parameters critical to product safety and quality. In this invention, input solution containing biologics is mixed with acid and pumped into a glass-packed column, where the solution is incubated for a time sufficient to inactivate enveloped viruses. Fluid pH is closely monitored by sensors and tightly regulated using a model-based, feedback control scheme. In addition, residence time distributions are periodically measured through inverse tracer experiments and used to adjust feed flow rates. The inventors demonstrate that the feedback control scheme enables fast and accurate regulation of the solution pH, with rapid startup and effective disturbance rejection. The inventors further determine that the system can correctly identify residence time changes and adjust feed flow rates to meet residence time setpoints. Such suppression to pH and residence time disturbances is essential for ensuring effective viral clearance with minimal degradation of product quality. Importantly, the inventors demonstrate that the system can effectively and consistently inactivate bacteriophage while maintaining pH and residence time setpoints, even over extended periods of time of 15 hours. Incorporating this invention into continuous biomanufacturing systems can increase productivity, improve product quality, and enhance patient safety.
Problem Addressed
Continuous manufacturing of biologics, such as antibodies, is attracting significant interest due to a number of benefits over traditional manufacturing systems, including lower costs, reduced processing times, and improved product consistency and quality. However, an important challenge during continuous biomanufacturing is the integration of effective, continuous viral clearance systems. Viral clearance techniques, including a low pH hold to inactivate enveloped viruses, are critical for minimizing viral contamination risks and ensuring patient safety. Previously invented approaches for integrating low pH holds into continuous processes lack sufficient control over key process parameters, such as operating pH and residence time. There is a need for tightly controlled, viral clearance systems that can be integrated into continuous biomanufacturing processes and ensure product quality and safety.
Advantages
- Low-cost, column-based viral inactivation system for use in continuous manufacturing of biologics
- Feedback control scheme allows for fast and accurate regulation over key control parameters, including effective suppression of pH and residence time disturbances
- System can effectively and consistently inactivate bacteriophage
- Technology provides tools for increasing productivity of biomanufacturing systems, improving product quality, and ensuring patient safety
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.