Synthesis of Covalent Protein Dimers that can Inhibit MYC-Driven Transcription
This technology utilizes automated flow protein synthesis to generate analogs of transcription factors. These synthetic molecules serve as a cancer treatment method by inhibiting transcription driven cancer cell proliferation.
Researchers
-
730697: 083474-023pc
Patent Cooperation Treaty | Published application
Figures
Technology
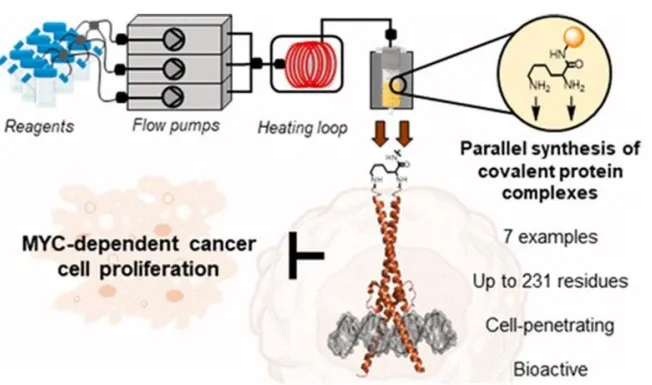
This technology provides a method for creating homologous or heterologous protein molecules wherein both molecules are at least 85% similar with either MYC, MAX, or Omomyc. These are produced via automated flow synthesis which uses tubes and channels to create a continual chemical reaction stream over a batch in a single reaction vessel. First, reagents are merged through flow pumps before activating heating loops. Next, the reagents are spread over a peptidyl resin which will support the protein structure synthesis. After, the two small molecules are connected via a linker to create the molecular structure, allowing the supportive resins to be cleaved from the final molecule.
These proteins can later be formulated with a pharmaceutically acceptable carrier such as water or buffered saline for easy administration to patients. Administration methods can be anything from injection to ingestion.
Problem Addressed
Transcription factors are proteins responsible for “turning on” and “off” certain genes which produce specific proteins. MYC is a transcription factor protein that can either bind with MAX, another transcription factor protein, to form heterologous molecules (MYC/MAX), or bind with itself to form homologous molecules (MAX/MAX). These molecules then compete for a shared DNA target site. MYC dysregulation and overexpression are observed in more than fifty percent of human cancers.
Current strategies to inhibit MYC activity rely on either stabilizing the MAX/MAX molecules or delivering structurally similar proteins to the area. This later approach utilizes Omomyc, an artificial miniprotein that mimics MYC and can compete for the DNA target site to inhibit tumor growth and prevent MYC/MAX-based gene transcription. Omomyc, MAX, and MYC all interact with one another to form different combinations; however, the dominating combination depends on the proteins’ cellular concentrations, which remain difficult to predict.
This technology improves upon these methods by preparing stable, well-defined analogs for the MYC and MAX protein molecules that can be directly administered to the patient. This provides a superior degree of control over the concentration and composition of the transcription factors, enabling the attenuation of MYC-driven gene transpiration and preventing the spread of cancerous cells.
Advantages
- Molecules are intrinsically able to penetrate and be internalized by cells.
- Method can rapidly produce engineered synthetic protein complexes.
- Compositions can be used to treat various cancers and other immune diseases including, but not limited to myasthenia gravis, psoriasis, pemphigus vulgaris, and atherosclerosis.
Publications
- Pomplun, Sebastian, Muhammad Jbara, Carly K. Schissel, Susana Wilson Hawken, Ann Boija, Charles Li, Isaac Klein, and Bradley L. Pentelute. 2021. "Parallel Automated Flow Synthesis of Covalent Protein Complexes That Can Inhibit MYC-Driven Transcription." ACS Central Science 7 (8): 1408-1418. https://doi.org/10.1021/acscentsci.1c00663.
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.