High Molecular Weight, Post-Translationally Modified Proteins Brushes Through Tyrosine Modification Chemistry
This invention enables the facile synthesis of high molecular weight mucins, the gel forming constituent and defining component of mucus. They are of interest to the defense and pharmaceutical industries because of their role in the human body’s primary defense against pathogens and toxins. Since mucus can decrease drug bioavailability, pharmaceutical companies may use mucins to test drug candidates. In the defense industry, there is considerable interest in using mucins to combat exogenous biological threats.
Researchers
-
high molecular weight, post-translationally modified protein brushes
United States of America | Granted | 9,963,492 -
high molecular weight, post-translationally modified proteins brushes through tyrosine modification chemistry
Patent Cooperation Treaty | Published application
Figures
Technology
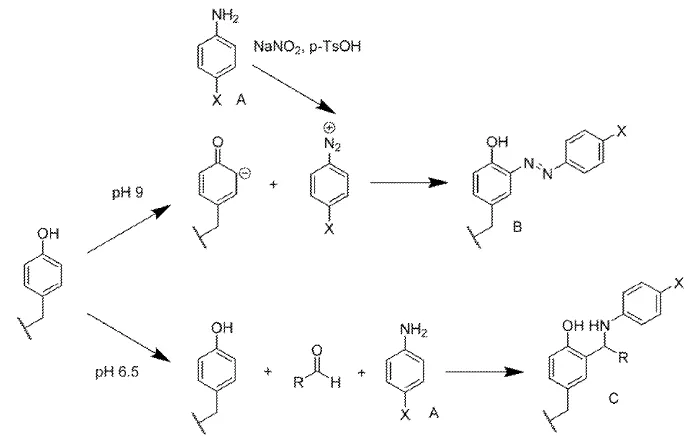
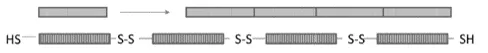
This invention generates artificially engineered respiratory mucin mimetic proteins that can be post-translationally modified through diazonium coupling to generate glycosylated products. The mucin mimetic proteins can then be extended via the oxidative coupling of cysteine residues to produce high molecular weight, densely functionalized mucin mimetic materials. The engineered mucin mimetic is comprised of the most frequent sequence of the variable number tandem repeats (VNTR) of respiratory mucins. Serines and threonines in these repeats have been replaced with tyrosines to facilitate diazonium-coupling and functionalization of the protein. Flanking the VNTR with cysteine residues facilitates protein-chain elongation through disulfide coupling.
Problem Addressed
Mucins are highly glycosylated, high molecular weight polymers that are very challenging to synthesize via chemical or molecular biology methods. Scraping pig stomachs yields microgram quantities of mucin and is the most common means of obtaining these polymers. However, synthetic methods exists to produce mucins, and are limited by their failure to generate highly glycosylated mucins that retain the physical and functional characteristics of native sources. This invention generates a protein series that mimics native respiratory mucins, generating high molecular weight and densely functionalizable mucin mimetic materials suitable for testing drug bioavailability.
Advantages
- Cost-effective post-translational modification to generate high molecular weight and densely functionalized mucin mimetics
- Mass functionalization of protein residues (rather than step-wise or individual residue functionalization)
Publications
Seifried, Brian M., Cao, James, and Olsen, Bradley D. "Multifunctional, High Molecular Weight, Post-Translationally Modified Proteins through Oxidative Cysteine Coupling and Tyrosine Modification." Bioconjugate Chemistry 29, no. 6 (2018): 1876-1884. doi: 10.1021/acs.bioconjchem.7b00834.
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.