Segregation Resistant Perovskite Oxides with Surfaces Modified by Binary Oxides and Heterogeneous Doping
This invention would be of interest to industries dealing with the application of perovskite oxide materials, including solid oxide fuel and electrolysis cells, catalysts for thermochemical splitting of water and carbon dioxide, oxygen permeation membranes and chemical looping, batteries, and magnetic and catalytic devices.
Researchers
-
segregation resistant perovskite oxides with surface modification
United States of America | Granted | 11,179,682
Figures
Technology
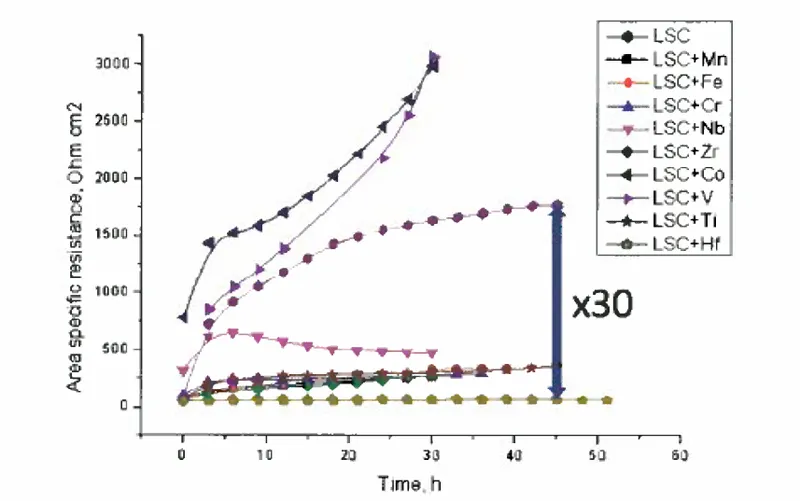
Perovskite oxides can be stabilized through the application of a thin film of oxidizing metal ions on the material's surface. The size mismatch, between the dopant and host cations and the associated elastic energy minimization push the larger or smaller dopants to free surface or interfaces. In addition, oxygen vacancies and the net positive charge at the surface of perovskite oxides drive the negatively charged dopants in the form of point defects to the surface. To minimize the electrostatic interactions that drive dopant segregation, this technology decreases the amount of the oxygen vacancies that can be equilibrated at the material surface. The surface positive charge is then reduced by putting more oxidizing metal cations at the surface, which lessens the amount of oxygen vacancies present, and thus, the electrostatic attraction of the dopants to the surface is weakened. By choosing a suitable surface additive cation, the stability of the perovskite oxide surface could be improved, and the final surface oxygen exchange reaction coefficient is increased up to 30 times.
Problem Addressed
Existing solid oxide fuel cells and electrolysis cells (SOFC, SOEC) have proven that the surface of such perovskite oxides are not stable. These surfaces undergo segregation and phase separation of dopant oxides at and above 400 degrees Celsius. The degradation of the chemical surface is harshly detrimental due to the consequent formation of a layer that blocks charge transfer. Choosing suitable dopants will therefore hinder the secondary phase segregation. This technology prevents elastic and electrostatic interactions, the key driving forces behind dopant segregation, by using a suitable surface additive cation that decreases the surface oxygen vacancy concentration at the surface.
Advantages
- Minimizes or completely avoids segregation and phase separation of dopant cations at the surface
- Improves chemical stability
- Able to function at temperatures above 300 degrees Celsius
- Improves surface charge transfer properties
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.