Crystal Structures of Human TorsinA and its Dystonia Mutant
This technology may be used as a treatment optionfor Early-Onset and other forms of Dystonia.
Researchers
-
crystal structures of human torsin-a and methods of determining and using the same
United States of America | Granted | 9,823,250
Figures
Technology
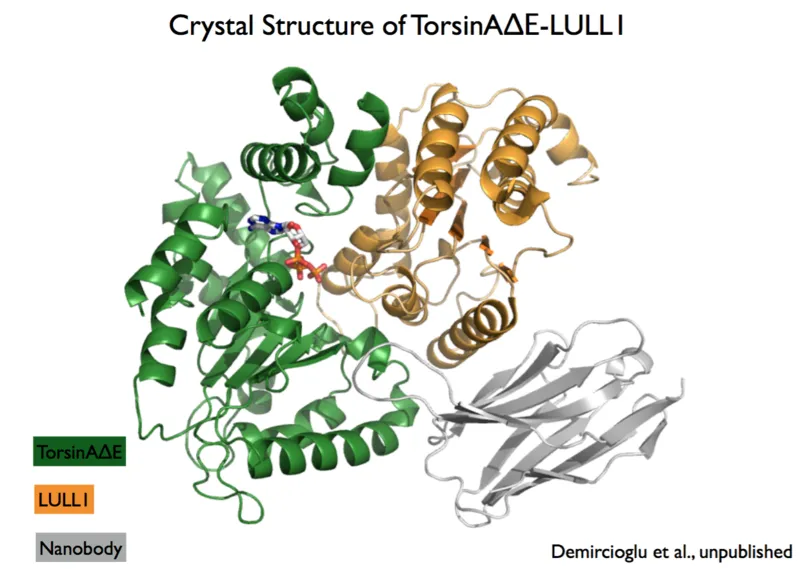
Early-onset, torsion dystonia (DYT1) is a genetic disease affecting an estimated 50,000 patients in the United Sates alone. It is an incurable and severely debilitating neuromuscular disease. A single glutamate deletion at position 302 or 303 of the protein TorsinA (TorsinADE302/303) is the primary cause of DYT1. TorsinA is an enzyme that resides in the endoplasmic reticulum of the cell, where it most likely functions in protein or membrane remodeling. TorsinA is activated by lamina-associated protein 1 (LAP1) and by luminal domain-like LAP1 (LULL1). The TorsinADE302/303 mutant weakens the binding of the activators LAP1/LULL1, which presumably presents the molecular basis for the disease. If binding to the activator can be restored, for examples by a small molecule, this may represent the drug needed to cure the disease. Here, a single-chain antibody fragment, or nanobody, called VHH-BS2, which specifically binds TorsinA and LULL1 has been found. Using this nanobody as a crystallization chaperone, both TorsinA-LULL1-VHH-BS2 and mutant TorsinADE302/303-LULL1-VHH-BS2 complexes were crystallized and the structures solved at very high resolution (1.4 Å). The ability to stabilize a weak interaction such as the one between mutant TorsinA and LULL1 is extremely rare and immediately suggests the possibility to provide a structural basis for rational drug design for DYT1.
Problem Addressed
A single-chain antibody fragment, known as nanobody, has been developed to serve as a crystallization chaperone for the structural characterization of TorsinA. The crystal structures of TorsinA as well as the Dystonia mutant TorsinADE302/303, both in complex with the activator LULL1, have been determined. These structures may lead to the development of a drug that can rescue the weakened TorsinADE302/303-activator interaction, with high potential of providing a cure for early-onset primary Dystonia.
Advantages
Crystal structures of wild type and dystonia mutant TorsinA protein complexes with nanobody and associated LULL1 activating protein have been determined at the nearly atomic resolution of 1.4 Å. The structures have a resolution that can directly be used for computational drug design. The TorsinA structure has been pursued for 20 years and only the use of the specific nanobody finally enabled crystallization.
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.