Electrochemically Tunable Affinity Separation (ETAS) of Organics from Water
Separation processes are of great importance in the chemical and environmental industries, accounting for 10-25% of the world’s energy consumption, and a third of total capital and operation costs in industrial plants. This invention demonstrates a separation technology with high energy efficiency and low environmental impact supporting downstream water sustainability and water economy.
Researchers
-
adsorbents, systems, and methods for separation of organic species from water
United States of America | Granted | 11,407,662
Figures
Technology
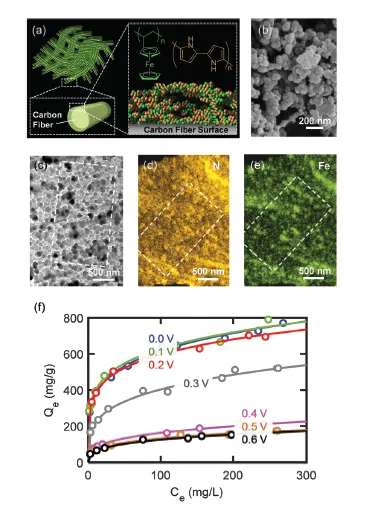
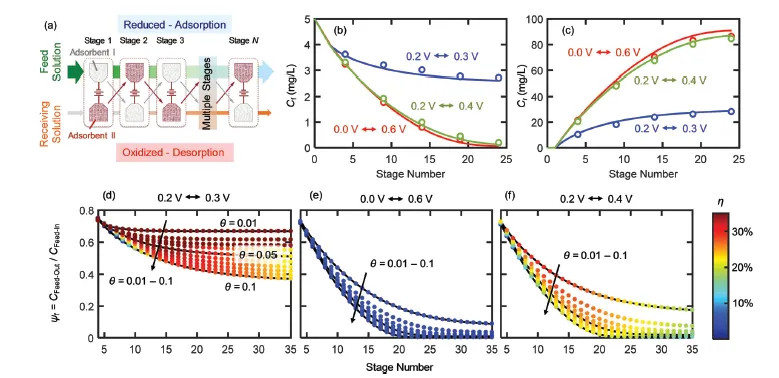
ETAS relies on the use of a stimulus-responsive adsorbent system that has surface hydrophobicity programmable by an electrical signal. Such an adsorbent displays an electrically controlled affinity toward neutral organic molecules, enabling the use of exquisite electrical swing to release and capture organics cyclically. The key to achieving ETAS resides in the development of multicomponent polymeric nanostructures that simultaneously exhibit an oxidation-state dependent affinity towards neutral organics, high porosity for sufficient adsorption capacity, and high conductivity to permit electrical manipulation. The ETAS absorbent system consists of a carbon cloth (CC) that serves as a flexible and robust conductive substrate, with a conformal coating of a polyvinylferrocene/polypyrrole (PVF/PPY) composite. The binary polymer film is fabricated via simultaneous electro-polymerization of pyrrole and electrodeposition of PVF. The ferrocene moieties in PVF render redox-tunable hydrophobicity while the conjugated PPY chains establish electron transport pathways to permit electrical control. Furthermore, through redox electrode simulations and theoretical calculations of electron transfer kinetics, a generalizable material design strategy is developed to simultaneously improve the separation degree and energetic efficiency during ETAS operation.
Problem Addressed
The global prevalence of contaminated water resources by organic pollutants, such as pesticides, dyes, pharmaceuticals, and endocrine disrupting compounds has raised concerns about potential deleterious effects on the environment and on human health. Electrochemically controlled processes, such as capacitive deionization are promising candidates for wastewater management and desalination. However, these processes rely on electrostatic interactions between the electrode and the target pollutant. These processes are not applicable to uncharged organic pollutants, which constitute the majority of industrial and municipal water contaminants, including dyes, pesticides, pharmaceuticals, and carcinogenic aromatics. High separation efficiencies for the removal of organics from water have been achieved using conventional processes such as adsorption, distillation, and solvent extraction, as well as more recent technologies such as advanced oxidation treatment and membrane separation. However, the overall separation processes inherently associated with these methods usually involve energy-intensive steps and/or environmentally unfriendly processes. This technology incorporates exquisite electrical control into separation of uncharged molecules from water and has higher energy efficiencies and lower environmental costs than established methods for separating neutral organics from water.
Advantages
- Separation of uncharged organic species from water
- Energy efficient due to operation at room temperature and pressure
- Environmentally responsible without using organic solvent and additives and does not generate secondary pollutants
- ETAS-integrated devices show modularity, portability and low-cost
- Highly flexible modulation of affinity for target pollutants with high spatiotemporal resolution to achieve intricate balance between the separation degree and the energetic efficiency
Publications
Xianwen Mao, et al. "Energetically Efficient Electrochemically Tunable Affinity Separation Using Multicomponent Polymeric Nanostructures for Water Treatment." Energy & Environmental Science (2018), 11: 2954-2963, https://doi.org/10.1039/C8EE02000K.
License this technology
Interested in this technology? Connect with our experienced licensing team to initiate the process.
Sign up for technology updates
Sign up now to receive the latest updates on cutting-edge technologies and innovations.